Embark on a captivating journey into the quantum realm with the Bohr model of hydrogen gizmo. This interactive simulation brings to life the groundbreaking atomic model, providing an immersive experience that deepens our understanding of atomic structure and behavior.
Prepare to unravel the mysteries of energy levels, electron transitions, and quantum numbers as we delve into the fascinating world of hydrogen atoms.
Bohr Model of Hydrogen Overview
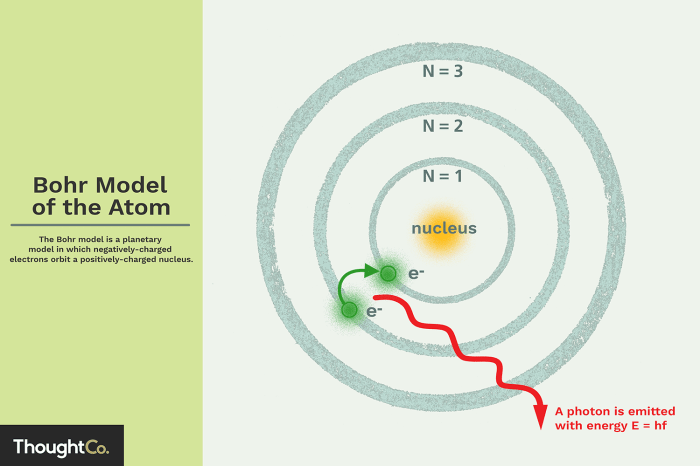
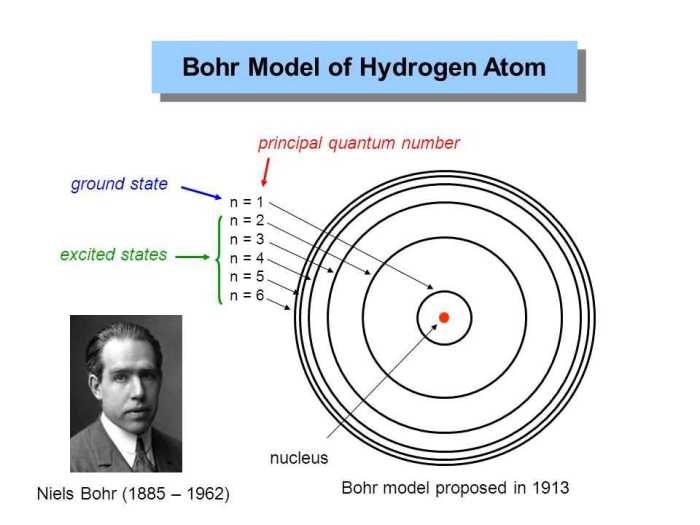
The Bohr model of hydrogen, proposed by Niels Bohr in 1913, is a simplified model of the hydrogen atom that describes the arrangement of electrons around the nucleus. It was a revolutionary concept that challenged the classical understanding of atomic structure and laid the foundation for modern quantum mechanics.
Bohr’s model is based on the idea that electrons occupy discrete energy levels around the nucleus. Each energy level is characterized by a specific radius and angular momentum. Electrons can transition between these energy levels by absorbing or emitting photons of light with a frequency corresponding to the energy difference between the levels.
Historical Context
Prior to Bohr’s model, the prevailing theory of atomic structure was the Rutherford model, which proposed that electrons orbit the nucleus in circular paths. However, this model could not explain the stability of atoms and the emission of specific wavelengths of light by hydrogen atoms.
Bohr’s model successfully addressed these shortcomings by introducing the concept of quantized energy levels and the idea that electrons could only occupy certain discrete orbits. This model laid the groundwork for understanding the behavior of electrons in atoms and provided a foundation for further advancements in quantum mechanics.
Energy Levels and Transitions
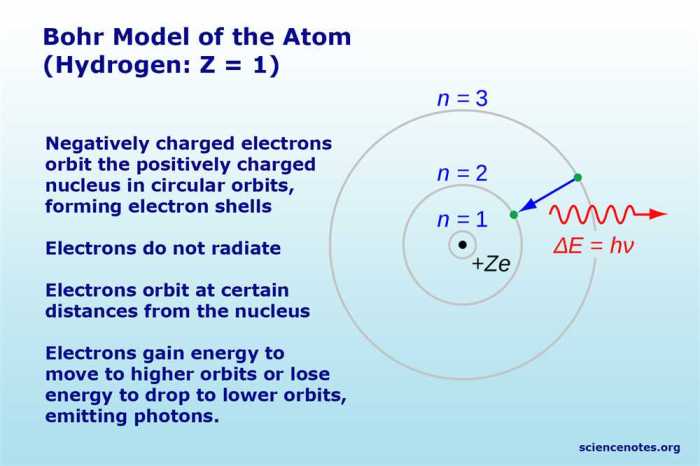
The Bohr model of the hydrogen atom describes the atom as a small, dense nucleus surrounded by electrons that orbit in specific energy levels. These energy levels are quantized, meaning that they can only exist at certain discrete values.
The energy of an electron in a particular energy level is given by the equation:
E =
13.6/n2eV
where:* E is the energy of the electron in electron volts (eV)
n is the principal quantum number, which can take on the values 1, 2, 3, …
The principal quantum number corresponds to the shell in which the electron is located. The lower the value of n, the closer the electron is to the nucleus and the lower its energy.
Electrons can transition from one energy level to another by absorbing or emitting photons. When an electron absorbs a photon, it moves to a higher energy level. When an electron emits a photon, it moves to a lower energy level.
The energy of the photon that is absorbed or emitted is equal to the difference in energy between the two energy levels involved in the transition.
Emission Spectrum
The emission spectrum of an atom is a series of lines that correspond to the different wavelengths of light that are emitted when electrons transition from higher energy levels to lower energy levels.
The emission spectrum of hydrogen is a series of lines in the visible and ultraviolet regions of the spectrum. The wavelengths of these lines are given by the Rydberg formula:
- /λ = RH(1/n 12
- 1/n 22)
where:* λ is the wavelength of the photon in meters
- R His the Rydberg constant (1.0973731×10 7m -1)
- n 1is the principal quantum number of the lower energy level
- n 2is the principal quantum number of the higher energy level
The emission spectrum of hydrogen is used to identify hydrogen atoms in stars and other objects.
Quantum Numbers
In the Bohr model of the hydrogen atom, electrons occupy specific energy levels, each characterized by a set of four quantum numbers: n, l, ml, and ms.
These quantum numbers provide a complete description of the electron’s state and determine its properties within the atom.
Principal Quantum Number (n)
The principal quantum number, n, describes the energy level of the electron. Higher values of n correspond to higher energy levels.
Azimuthal Quantum Number (l)
The azimuthal quantum number, l, describes the shape of the electron’s orbital. It can take values from 0 to n-1, where n is the principal quantum number.
Magnetic Quantum Number (ml)
The magnetic quantum number, ml, describes the orientation of the electron’s orbital in space. It can take values from -l to +l, including zero.
Spin Quantum Number (ms)
The spin quantum number, ms, describes the intrinsic spin of the electron. It can take values of +1/2 or -1/2, representing the two possible spin states of the electron.
Rydberg Formula and Spectral Lines: Bohr Model Of Hydrogen Gizmo
The Rydberg formula is an equation that describes the wavelengths of spectral lines emitted by hydrogen atoms. It was developed by the Swedish physicist Johannes Rydberg in 1888.
The Rydberg formula is given by: $$1/\lambda = R_H (\frac1n_f^2 – \frac1n_i^2)$$ where:
- $1/\lambda$ is the wavenumber of the spectral line (in cm -1)
- $R_H$ is the Rydberg constant (109,73731 cm -1)
- $n_f$ is the final energy level of the electron
- $n_i$ is the initial energy level of the electron
The Rydberg formula can be used to calculate the wavelengths of spectral lines emitted by hydrogen atoms when an electron transitions from one energy level to another. For example, the wavelength of the red spectral line emitted by hydrogen is 656.3 nm.
This corresponds to a transition from the n=3 energy level to the n=2 energy level.
The Rydberg formula is a powerful tool for understanding the structure of atoms and the emission of light. It has been used to measure the wavelengths of spectral lines with great accuracy and to determine the energy levels of electrons in atoms.
Limitations and Extensions
The Bohr model, despite its success in explaining the hydrogen atom, has limitations. It fails to explain several key phenomena, such as the fine structure of spectral lines and the behavior of atoms in electric and magnetic fields.
To overcome these limitations, the Bohr model was extended by subsequent models, including the quantum mechanical model developed by Erwin Schrödinger and Werner Heisenberg. These models incorporate wave-particle duality and the uncertainty principle, providing a more complete description of atomic structure and behavior.
Role in the Development of Quantum Mechanics, Bohr model of hydrogen gizmo
The Bohr model played a crucial role in the development of quantum mechanics. It provided the first concrete evidence for the quantization of energy levels in atoms and laid the foundation for the development of quantum theory.
The Bohr model’s success in explaining the hydrogen atom demonstrated that classical physics could not fully describe atomic phenomena. It paved the way for the development of quantum mechanics, which provides a more accurate and comprehensive description of the atomic world.
Applications
The Bohr model has numerous practical applications in various fields, including spectroscopy and astrophysics. In spectroscopy, it aids in understanding the emission and absorption spectra of atoms, providing insights into their electronic structure and energy levels. This knowledge finds use in diverse areas, such as chemical analysis, material characterization, and medical diagnostics.In
astrophysics, the Bohr model contributes to comprehending the behavior of atoms in celestial bodies. It helps explain the spectral lines observed in stellar spectra, providing valuable information about the composition, temperature, and other characteristics of stars and galaxies. Furthermore, the model plays a role in understanding phenomena like atomic transitions and energy level interactions in astrophysical environments.
Gizmo Simulation
The Gizmo simulation provides an interactive environment to visualize and explore the Bohr model of hydrogen. It allows users to manipulate various parameters, such as the energy levels of electrons and the angular momentum, to observe the corresponding changes in the electron’s behavior.
The Gizmo simulation features a graphical representation of the hydrogen atom, with electrons orbiting the nucleus in discrete energy levels. Users can select different energy levels to visualize the electron’s wave function and calculate its energy. They can also apply an external magnetic field to observe the Zeeman effect, which splits the energy levels due to the interaction between the electron’s magnetic moment and the magnetic field.
Simulation Features
- Visualization of electron orbits in different energy levels
- Calculation of electron energy and wave function
- Application of external magnetic field to observe the Zeeman effect
- Measurement of energy transitions and emission wavelengths
- Exploration of the relationship between energy levels and spectral lines
Gizmo Activities
The Gizmo simulation provides a range of activities that allow students to explore the Bohr model of hydrogen and its implications.
To set up the simulation, students can select the “Energy Levels” tab and adjust the principal quantum number (n) to observe the corresponding energy levels and electron transitions. They can also toggle the “Show Wave Function” option to visualize the probability distribution of the electron in each energy level.
Running the Simulation
- Students can initiate the simulation by clicking the “Play” button. The electron will transition between energy levels, emitting photons with specific wavelengths.
- By adjusting the “Time Scale” slider, students can control the speed of the simulation, allowing them to observe the transitions in detail.
- The “Data Table” tab records the energy levels, wavelengths, and frequencies of the emitted photons, enabling students to analyze the relationship between these quantities.
Gizmo Analysis
The Gizmo simulation provides a valuable tool for analyzing the Bohr model and its predictions. It allows users to manipulate various parameters, such as the principal quantum number, and observe the corresponding changes in the energy levels, wavelengths, and spectral lines of the hydrogen atom.
This interactive approach enables students to gain a deeper understanding of the model and its implications.
Benefits of Using the Gizmo Simulation
-
-*Visual Representation
The simulation provides a visual representation of the Bohr model, making it easier for students to visualize the energy levels and transitions of electrons.
-*Interactive Exploration
Users can interactively change the principal quantum number and observe the corresponding changes in the energy levels, wavelengths, and spectral lines. This allows them to explore the model in a hands-on manner.
The Bohr model of the hydrogen gizmo is a valuable tool for visualizing the atom. Just like the NFPA model code of ethics provides guidelines for ethical behavior in the fire protection industry, the Bohr model helps us understand the structure of atoms.
Both models are essential for their respective fields.
-*Quantitative Analysis
The simulation provides numerical data on the energy levels, wavelengths, and spectral lines, which can be used for quantitative analysis and comparison with experimental data.
Limitations of Using the Gizmo Simulation
-
-*Simplified Model
The Gizmo simulation is a simplified representation of the Bohr model and does not account for all of its complexities, such as electron spin and relativistic effects.
-*Limited Applicability
The simulation is only applicable to the hydrogen atom and cannot be used to analyze other atoms or molecules.
-*Potential Misconceptions
The simulation can lead to misconceptions if students do not fully understand the underlying physics of the Bohr model and its limitations.
Conclusion
The Bohr model of hydrogen laid the foundation for understanding the structure of atoms and the quantization of energy. It introduced the concept of discrete energy levels and explained the emission and absorption of light by hydrogen atoms. The model’s significance lies in its ability to accurately predict the wavelengths of light emitted by hydrogen atoms, confirming the wave-particle duality of light.
The Gizmo simulation provides an interactive and engaging way to explore the Bohr model. It allows students to visualize the energy levels of the hydrogen atom and observe the transitions between these levels, which are responsible for the emission and absorption of light.
By adjusting the energy of the incoming photon, students can observe how the electron transitions to different energy levels and emits or absorbs light accordingly.
Contributions of the Gizmo Simulation
- Visualization of energy levels: The Gizmo simulation provides a clear visual representation of the energy levels of the hydrogen atom, making it easier to understand the concept of quantization.
- Observation of transitions: Students can observe the transitions between energy levels in real time, gaining a better understanding of how electrons absorb and emit light.
- Prediction of wavelengths: The Gizmo simulation allows students to calculate the wavelength of light emitted or absorbed during transitions, reinforcing the relationship between energy and wavelength.
- Interactive exploration: The interactive nature of the Gizmo simulation allows students to experiment with different scenarios and deepen their understanding of the Bohr model.
FAQ Resource
What is the significance of the Bohr model of hydrogen?
The Bohr model was a groundbreaking theory that introduced the concept of quantized energy levels within atoms. It laid the foundation for understanding atomic structure and behavior, paving the way for the development of quantum mechanics.
How does the Gizmo simulation enhance the learning experience?
The Gizmo simulation provides an interactive platform for visualizing the Bohr model. Learners can manipulate energy levels, observe electron transitions, and analyze spectral lines, fostering a deeper understanding of the atomic processes.
What are the limitations of the Bohr model?
While the Bohr model revolutionized our understanding of atomic structure, it has certain limitations. It does not fully account for the wave-particle duality of electrons or the behavior of atoms with multiple electrons.