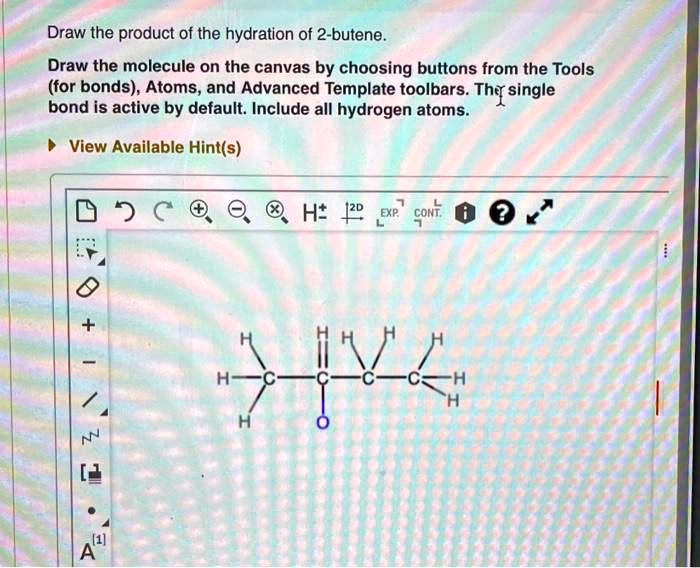
Draw the product of the hydration of 2 butene – Draw the product of the hydration of 2-butene, a captivating journey into the realm of organic chemistry, where we unravel the intricacies of a fundamental reaction. This process, essential for understanding the behavior of alkenes, unveils the mechanisms, regioselectivity, stereoselectivity, and diverse applications of hydration.
The hydration of 2-butene, a seemingly straightforward reaction, reveals a wealth of complexities that challenge our understanding of chemical reactivity. Through detailed reaction schemes, we delve into the mechanistic pathways, exploring the factors that govern regio- and stereoselectivity, ultimately leading to a deeper comprehension of alkene hydration.
Hydration of 2-Butene
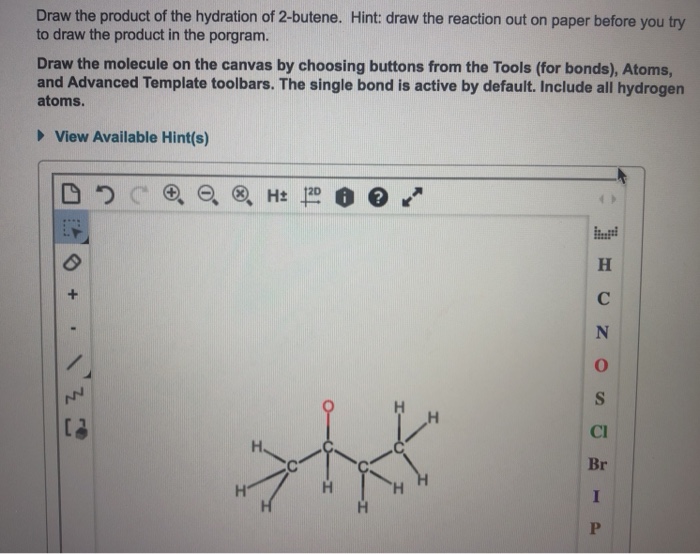
Hydration is a chemical reaction in which water is added to a molecule. In the case of 2-butene, hydration results in the formation of 2-butanol.
The mechanism of hydration of 2-butene involves the addition of a water molecule to the double bond of the alkene. This addition occurs in a Markovnikov fashion, meaning that the water molecule adds to the carbon atom that is already bonded to the most hydrogen atoms.
The resulting carbocation is then attacked by a hydroxide ion to form the alcohol.
The reaction scheme for the hydration of 2-butene is as follows:
Regioselectivity and Stereoselectivity
The hydration of 2-butene is a regioselective reaction, meaning that the water molecule adds to the double bond in a specific way. In this case, the water molecule adds to the carbon atom that is already bonded to the most hydrogen atoms.
This regioselectivity is due to the fact that the carbocation that is formed in the first step of the reaction is more stable when it is formed on the carbon atom that is already bonded to the most hydrogen atoms.
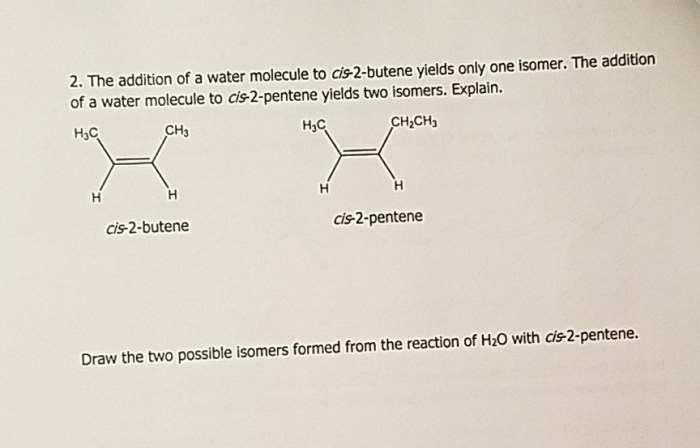
The hydration of 2-butene is also a stereoselective reaction, meaning that the water molecule adds to the double bond in a specific way. In this case, the water molecule adds to the double bond in a syn fashion, meaning that the hydroxyl group and the hydrogen atom that is added to the double bond are on the same side of the molecule.
This stereoselectivity is due to the fact that the transition state for the reaction is more stable when the hydroxyl group and the hydrogen atom that is added to the double bond are on the same side of the molecule.
The regio- and stereoselective products of the hydration of 2-butene are shown below:
Factors Affecting Hydration, Draw the product of the hydration of 2 butene
The hydration of 2-butene is affected by a number of factors, including temperature, solvent, and the presence of a catalyst.
The rate of hydration of 2-butene increases with increasing temperature. This is because the activation energy for the reaction is lower at higher temperatures. The solvent also affects the rate of hydration of 2-butene. The rate of hydration is faster in polar solvents than in nonpolar solvents.
This is because the polar solvent molecules solvate the ions that are formed in the reaction, which makes the reaction more favorable.
The presence of a catalyst can also affect the rate of hydration of 2-butene. Catalysts are substances that increase the rate of a reaction without being consumed in the reaction. The most common catalyst for the hydration of 2-butene is sulfuric acid.
Sulfuric acid protonates the double bond of the alkene, which makes the alkene more reactive towards attack by water.
Applications of Hydration
The hydration of 2-butene is a versatile reaction that can be used to synthesize a variety of organic compounds. One of the most important applications of the hydration of 2-butene is the synthesis of 2-butanol. 2-Butanol is a valuable industrial solvent that is used in a variety of applications, including the production of paints, coatings, and adhesives.
The hydration of 2-butene can also be used to synthesize a variety of other organic compounds, including 2-butanone, 2-butene-1,4-diol, and 2-butyne. These compounds are used in a variety of applications, including the production of plastics, pharmaceuticals, and fragrances.
User Queries: Draw The Product Of The Hydration Of 2 Butene
What is the mechanism of hydration of 2-butene?
The hydration of 2-butene proceeds via a two-step electrophilic addition mechanism. The first step involves the addition of a proton to the double bond, forming a carbocation intermediate. In the second step, a water molecule adds to the carbocation, forming the final product.
What are the factors that affect the regioselectivity of the hydration of 2-butene?
The regioselectivity of the hydration of 2-butene is primarily determined by the stability of the carbocation intermediate. The more stable carbocation will be formed preferentially, leading to the major product.
What are the applications of the hydration of 2-butene?
The hydration of 2-butene is a versatile reaction with numerous applications in organic synthesis. It is commonly used for the production of alcohols, ethers, and other organic compounds.